High-throughput protein production in E. coli, purification and interaction
This PBSIM facility uses its own high-throughput method to perform all the necessary steps from cloned genes to purified proteins. The team develops strategies for its own research projects that are constantly evolving and enriching its service portfolio for its collaborators and clients.
The protein production and purification service is open to the comunity since 2004. It is recognized by several national labels: IBiSA, AMU and FRISBI. It offers a portfolio of automated protocols (96 or 384 format) which cover all the stages of cloning, protein production and purification as well as the in vitro study of protein-protein, protein-peptide or protein-DNA interactions. Since its launch, more than 10,000 proteins have been produced for our customers, collaborators or partners from national (13 ANR as partner or subcontractor) or international (12 European networks) networks. The facility is co-author of more than eighty publications and regularly teaches some of its protocols in local (Polytech, Plinius), national (FRISBI, INSERM) and international (EMBO) practical training courses.
Team contacts
Services
- Gene cloning
- Expression screening: optimization of E. coli culture conditions to improve protein solubility
- Protein refolding
- Purification
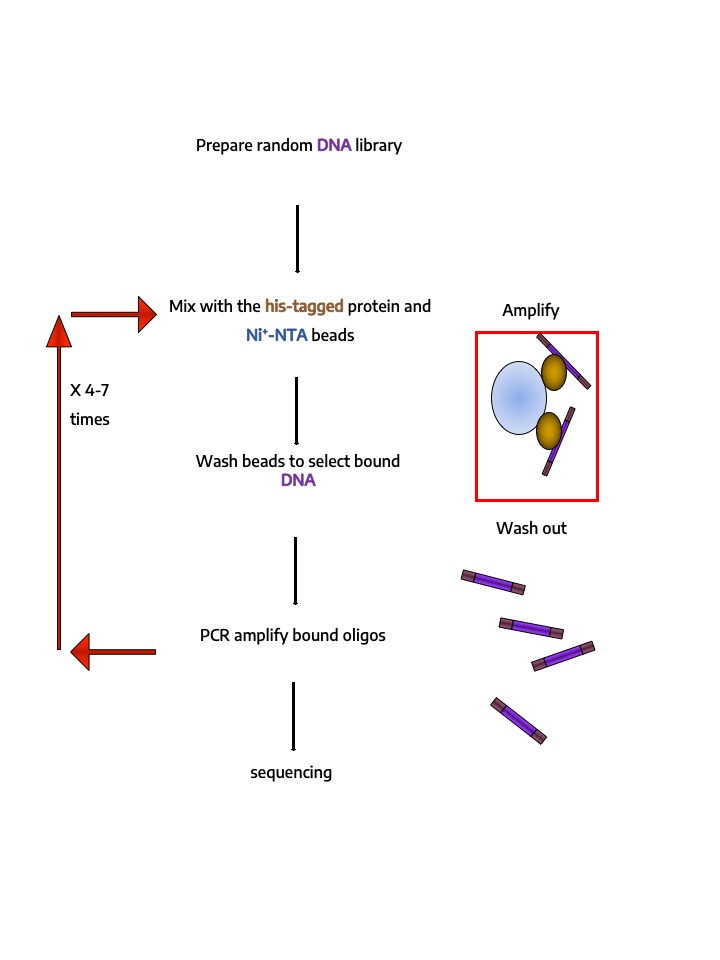
- In vitro Identification and quantification of protein-protein, protein-peptide (pull-down, holdup, Co-IP) or protein-DNA interactions (HT-SELEX)
Specific Equipment
- Two pipetting robots equipped with a 96-channel head EVO®(Tecan)
- Two 96/384 LabChip® GXII (Perkin Elmer) capillary electrophoresis systems
- One Hydrospeed® 96/384 plate-washer with vacuum or magnetic modules (Tecan)
- One Pherastar® FSX (BMG LabTech) plate-reader for 96/384/1536 samples in UV/Fluorescence/Luminescence/HTRF
- One Microtron® (Infors) incubator for 48 DeepWells 24/96 bacterial cultures (Infors)
- Four Aktä®Xpress (Cytiva) purification systems (4×4 modules) at 4°C
- One photopolymer resin Saturn® (Elegoo) 3D printer
Methods development
The vast majority of protocols used for the services have been developed, validated and published by the facility. We develop new protocols when necessary for our research projects or at the request of our customers. We collaborate and validate new methods with our colleagues from the P4EU network (>100 facilities) of which we are one of the five founding members (2011).
All our analytical protocols are automated in microplate 96 or 384 format (Tecan robot) or on the AKTA Xpress (Cytiva) for large scale protein purification (mg).
Expression screening: optimization of culture conditions to determine and improve protein solubility level in E. coli
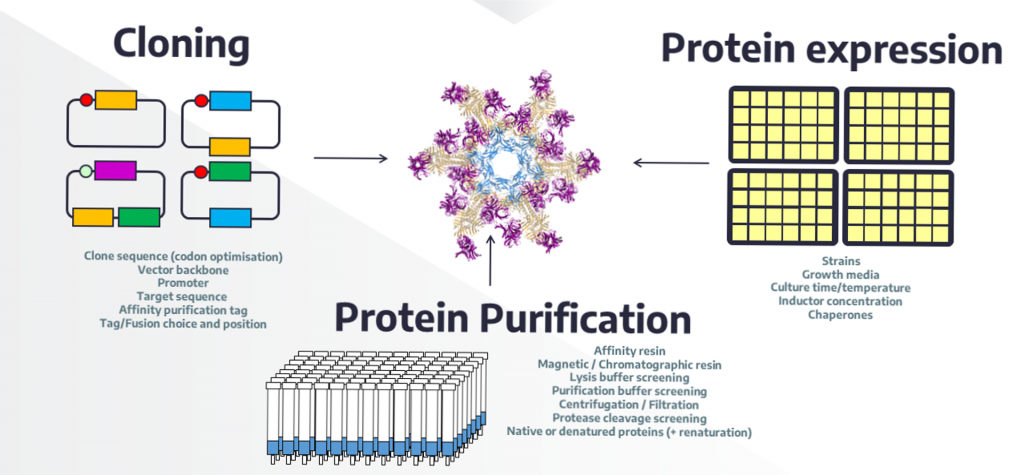
High-throughput expression screening: Parameters that can alter protein solubility and quality
Vincentelli et al. (2011) Methods, Vincentelli. R and Romier. C (2013) Curr Opin Struct Biol, N. Saez and Vincentelli. R (2014) Methods Mol Biol.
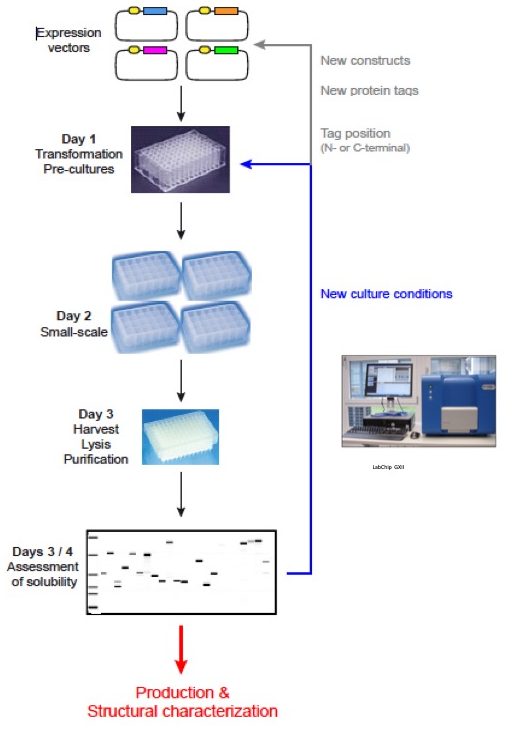
Protein renaturation
Protein purification
The scale of production and purification is adapted according to the needs of the project:
“Analytical” or “semi-preparative” culture and purification: culture in DeepWell 24/96/384 and purification on filter plates 24/96/384. This scale is used for “expression screening” or for the production of protein libraries. The largest library of purified proteins to date included 5,000 recombinant animal toxins.
- Culture and “preparative” purification : culture in a 2-liter flask and purification on AKTA Xpress (Cytiva). All the purification techniques are possible (affinity, gel-filtration, ion exchange, etc.). This scale is used for project containing one to a few tens of proteins at the milligram scale.
High-throughput In vitro identification and quantification of protein-protein, protein-peptide (pull-down, holdup, Co-IP) or protein-DNA (HT-SELEX) interactions.
High-throughput In vitro identification and quantification of protein-protein, protein-peptide (pull-down, holdup, Co-IP) or protein-DNA (HT-SELEX) interactions.
Research Projects and collaborations
The facility is involved as a partner, participant, or service provider (CRO) in research projects with private or public funds. Most of these projects require innovative protocols and this is the main driving force behind the evolution of the platform’s portfolio. The facility has participated in twelve European networks (FP5, FP6, FP7, H2020 funding) and thirteen ANRs (ANR is the French project research funding agency). Most of the projects stop with the end of funding, but some have become long-term collaborations funded either by several ANRs (or successive European networks), or by our own funds.
Animal toxin Purification
Since 2010, the platform has developed various strategies to produce animal toxins in oxidized form in E. coli. These methods have been refined and applied on a large scale to thousands of toxins for the VENOMICS project (FP7, 2012-2015).
Since 2021, the facility has been one of the five European partners of the H2020 Addovenom project. This project seeks to develop a new treatment against snakebites which kill, even in the 21st century, more than 140,000 people every year.
For more informations about snakebites, you can see the documentary “Minutes To Die”
The facility is a member of the European COST Euven toxin network.
PDZ domains purification and interaction
This project stems from a long collaboration between the facility and the team of Dr. G. Travé at the IGBMC which began with the ANR EPI-HPV-3D (2007-2010). This collaboration is still very active.
We have developed and improved together a library of clones, a protocol for the production of human PDZ domains as well as a high-throughput protocol for the identification and quantification of PDZ-PBM interactions that can now determine thousands affinities/day and is applicable to a very large number of protein-protein or protein-peptide interactions. The PDZ libraries and these methods have been used by several European teams, in particular by members of the ITN H2020 PDZNet project, whose facility was one of the associated laboratories.
CAZYmes purification
The facility has produced and purified more than 2.000 CAZYmes for the Glycogenomics team (Bernard Henrissat and Nicolas Terrapon) and its collaborators, mainly within the framework of three successive ANRs (1000 CAZymes, Pulmarin and ODE) and the NovoNordisk foundation CAZAI project.
Current grants
Coordinator :
ANR Full-PDZ-PBM-HPVE6 (2023-2025). Partner : Dr. G. Travé at the IGBMC.
Partner :
European : Addovenom (2021-2025)
NovoNordisk foundation : CAZAI (2023-2027)
ANR AirMN (2021-2025)
Participant :
ANR Rha (2024-2027) and ODE (2021-2024)
Collaboration and services
The collaborations and services mentioned below correspond to the period 2016-2021. When a link exists, it refers to the last common publication over the period.
National
- Marie-Noëlle Rosso, Giuliano Sciara
- BBF, Marseille
- Rémi Lasserre, Phillipe Naquet, Beatrice NalRogier, Philippe Pierre
- CIML, Marseille
- Jean-Paul Borg, Patrick Fourquet, Pascale Zimmermann
- CRCM, Marseille
- Manos Mavrakis, Jérome Wenger
- Fresnel Institute, Marseille
- André Le Bivic, Laurent Kodjabachian, Alphée Michelot, Aziz Mogrich, Thomas Rival
- IBDML, Marseille
- Félix Rico, Olivier Théodely
- LAI, Marseille
- François Alberto, Tâm Mignot
- LCB, Marseille
- Andreas Zanzoni, Christine Brun
- TAGC, Marseille
- Bruno Coutard
- UVE, Marseille
- Jérome Martin, François Torney
- Biogemma/Limagrain, Chappes
- Nicolas Gilles
- CEA, Saclay
- William Helbert
- Cermav, Grenoble
- Patrick Lemaire
- CRBM, Montpellier
- Stéphanie Cabantous
- CRCT, Toulouse
- Christophe Romier, Arnaud Poterszman, Gilles Travé
- IGBMC, Strasbourg
- Pascale Cossart, Nicolas Wolff
- Pasteur Institute, Paris
- Gérard Lambeau
- IPMC, Valbonne
International
- Kim Remans
- EMBL, Heidelberg, Germany
- Carlos Fontes
- NZYTech, Lisbon, Portugal
- Cherith Reid
- Randox, Crumlin, UK
- Christiane Berger-Schaffitzel, Imre Berger
- University of Bristol, UK
- Henrick Clausen, Kristian Strømgaard
- University of Coppenhagen, Denmark
- Douwe van Sinderen
- University College Cork, Ireland
- László Nyitray
- University Eötvös Loránd, Budapest, Hungary
- Edwin De Pauw, Loic Quinton
- Université de Liège, Belgium
- Stephane Mesnage
- University of Sheffield, UK
- Jonas V. Schaefer
- University of Zurich, Switzerland
- Johnny Habchi
- Wren Therapeutics Ltd, Cambridge, UK
- Robert Allan Heinzen
- NIH Rocky Mountain, Montana, USA
- Sarel-Jacob Fleishman
- Weizmann Institute, Rehovot, Israël
Access
Academic and private companies
Cost
Coming soon
Fundings








Publication
The development of methods and the work carried out on the facility for our collaborators has given rise to more than sixty publications as co-authors, including articles in journals with high impact factors such as: Cell, Nature Methods, Nature Microbiology, Nature Communication, PNAS. The team also wrote five book chapters and coordinated the editing of the first book in the “Methods in Molecular Biology” series dedicated to “High-Throughput Protein Production and Purification” (2019).

Members
News
September 13, 2022 ADDovenom Year 2 annual meeting in Marseille

October 5, 2021 First ADDovenom annual meeting kicks off in Bristol