Équipe
Désordre Structural et Reconnaissance Moléculaire
L’objectif du groupe est l’identification, la caractérisation et l’élucidation du rôle fonctionnel de protéines ou régions protéiques intrinsèquement désordonnées dans des systèmes biologiques pertinents en termes de santé humaine.
En particulier, l’équipe s’intéresse aux protéines du complexe réplicatif de virus pathogènes humains majeurs, tels que le virus de la rougeole et le virus Nipah.
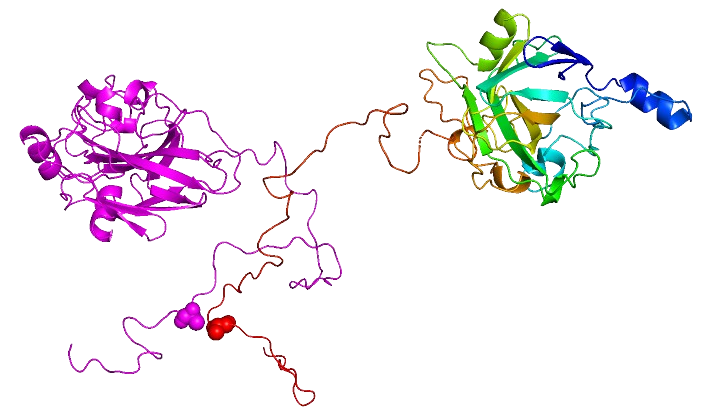
Au cours de ces vingt dernières années, le paradigme «structure-fonction » a été remis en cause par la découverte des protéines intrinsèquement désordonnées (IDPs), à savoir des protéines dépourvues de structure secondaire et tertiaire stables en conditions physiologiques de pH et salinité et en l’absence d’un partenaire ou ligand. Cependant, elles sont fonctionnelles et très répandues dans le monde du vivant. Le groupe a joué un rôle clé en découvrant que la nucléoprotéine (N) et la phosphoprotéine (P) du virus de la rougeole possèdent de longues régions désordonnées (allant jusqu’à 300 résidus), et a ensuite étendu ces résultats aux protéines N et P des virus Nipah et Hendra, deux pathogènes humains de classe 4.
Cette découverte ouvre de nombreuses perspectives intéressantes d’un point de vue fondamental mais aussi d’un point de vue thérapeutique. L’originalité et les points forts de ces travaux sont liés à leur multidisciplinarité de par l’intégration des approches bioinformatiques, biochimiques, biophysiques et structurales.
Plus récemment le groupe a commencé à s’intéresser aux phénomènes de séparation et transition de phase médiés par des régions protéiques intrinsèquement désordonnées. Les phénomènes de séparation de phase liquide-liquide (LLPS) constituent un nouveau paradigme en biologie. Ils sont à l’origine de la formation des organites sans membranes mais également des usines virales chez les Mononégavirales. Ces condensats permettent une régulation spatio-temporelle fine des activités cellulaires. Ils peuvent subir une « maturation » et nucléer des fibres de type amyloïde.
Les recherches du groupe s’articulent autours des trois axes suivants:
- Séparation ou transition de phase et fibrillation des protéines V et W des virus Nipah et Hendra. Ces protéines jouent un rôle clé dans l’évasion de la réponse immunitaire innée de l’hôte. Les recherches, réalisées dans la cadre du projet ANR HENIPHASE et d’un projet financé par le CEFIPRA (collaboration avec Samrat Mukhopadyay, IISER, Mohali, India), visent à (i) élucider les déterminants moléculaires de la fibrillation de ces protéines, (ii) élucider le rôle fonctionnel de ces phénomènes dans le contexte cellulaire, (iii) déterminer la structure de ces fibres par cryo-EM, et (iv) évaluer le potentiel antiviral d’inhibiteurs de la fibrillation.
- Séparation de phase liquide-liquide (LLPS) conduisant à la formation des usines virales chez les paramyxovirus. Les recherches, conduites dans le cadre du projet ANRS PEPR MIE 2023 NIPAH-LISA impliquant au total 8 équipes de recherche, visent à développer des approches antivirales innovantes ciblant la formation et/ou la dynamique des usines virales des virus Nipah, de la rougeole et parainfluenza humain de type 3.
- Séparation de phase liquide-liquide (LLPS) par les facteurs de transcription de la famille Hox de la Drosophile (collaboration avec Yacine Graba, IBDM, Marseille dans le cadre du projet Amidex BLANC Rercherche IDRHOX) et d’Arabidopsis thaliana (collaboration avec Thierry Desnos, BIAM, Cadarache, dans le cadre d’un projet soutenu par l’IM2B).
Contacter le groupe
Domaines de recherche
- protéines ou régions protéiques intrinsèquement désordonnées
- séparation de phase et fibrillation
- paramyxovirus
Membres
Publications
The C-terminal intrinsically disordered region of a fungal LPMO binds copper and displays anti-fungal properties
Ketty C Tamburrini, Koar Chorozian, Clarisse Roblin, Aurore Labourel, Mireille Haon, Sacha Grisel, Evangelos Topakas, Michael Lafond, Bruno Guigliarelli, Gaston Courtade, Sonia Longhi, Jean-Guy Berrin
Cysteine Redox State Governs the Condensation Pathway of Hendra Virus W Protein and Differentially Impacts Type I IFN and NF-κB signaling
Frank Gondelaud, Alexandre Lalande, Giulia Pesce, Christophe Bignon, Denis Ptchelkine, Pierre-Yves Lozach, Denis Gerlier, Cyrille Mathieu, Sonia Longhi
Transient non-local interactions dominate the dynamics of measles virus NTAIL
Lillian Otteson, Gabor Nagy, John Kunkel, Gerdenis Kodis, Lars Bock, Christophe Bignon, Sonia Longhi, Wenwei Zheng, Helmut Grubmüller, Andrea Vaiana, Sara Vaiana
Communications Chemistry 8:298 (2025)10.1038/s42004-025-01682-0
Publications de l'équipe
The C-terminal intrinsically disordered region of a fungal LPMO binds copper and displays anti-fungal properties
Ketty C Tamburrini, Koar Chorozian, Clarisse Roblin, Aurore Labourel, Mireille Haon, Sacha Grisel, Evangelos Topakas, Michael Lafond, Bruno Guigliarelli, Gaston Courtade, Sonia Longhi, Jean-Guy Berrin
Cysteine Redox State Governs the Condensation Pathway of Hendra Virus W Protein and Differentially Impacts Type I IFN and NF-κB signaling
Frank Gondelaud, Alexandre Lalande, Giulia Pesce, Christophe Bignon, Denis Ptchelkine, Pierre-Yves Lozach, Denis Gerlier, Cyrille Mathieu, Sonia Longhi
Transient non-local interactions dominate the dynamics of measles virus NTAIL
Lillian Otteson, Gabor Nagy, John Kunkel, Gerdenis Kodis, Lars Bock, Christophe Bignon, Sonia Longhi, Wenwei Zheng, Helmut Grubmüller, Andrea Vaiana, Sara Vaiana
Communications Chemistry 8:298 (2025)10.1038/s42004-025-01682-0
When artificial intelligence meets protein research
Sonia Longhi, Salvador Ventura, Sandra Macedo-Ribeiro, Leandro G Radusky, Jovana Kovačević, R. Gonzalo Parra, Miguel A Andrade-Navarro, Andrey V Kajava, Zuzana Bednáriková, Alexander Monzon, Rita Vilaça
Open Research Europe 5:185 (2025)10.12688/openreseurope.20628.1
A conserved motif in Henipavirus P/V/W proteins drives the fibrillation of the W protein from Hendra virus
Frank Gondelaud, Christophe Bignon, Denis Ptchelkine, Frédéric Carrière, Sonia Longhi
Protein Science 34 (2025)10.1002/pro.70085
Unraveling the molecular grammar and the structural transitions underlying the fibrillation of a viral fibrillogenic domain
Frank Gondelaud, Julien Leval, Lisha Arora, Anuja Walimbe, Christophe Bignon, Denis Ptchelkine, Stefania Brocca, Samrat Mukhopadyay, Sonia Longhi
Protein Science 34:e70068 (2025)10.1002/pro.70068
Unraveling Liquid–Liquid Phase Separation (LLPS) in Viral Infections to Understand and Treat Viral Diseases
Marie Galloux, Sonia Longhi
International Journal of Molecular Sciences 25:6981 (2024)10.3390/ijms25136981
PED in 2024: improving the community deposition of structural ensembles for intrinsically disordered proteins
Hamidreza Ghafouri, Tamas Lazar, Alessio del Conte, Luiggi G Tenorio Ku, Maria C Aspromonte, Pau Bernadó, Belén Chaves-Arquero, Lucia Beatriz Chemes, Damiano Clementel, Tiago N Cordeiro, Carlos A Elena-Real, Michael Feig, Isabella C Felli, Carlo Ferrari, Julie D Forman-Kay, Tiago Gomes, Frank Gondelaud, Claudiu C Gradinaru, Tâp Ha-Duong, Teresa Head-Gordon, Pétur O Heidarsson, Giacomo Janson, Gunnar Jeschke, Emanuela Leonardi, Zi Hao Liu, Sonia Longhi, Xamuel L Lund, Maria J Macias, Pau Martin-Malpartida, Davide Mercadante, Assia Mouhand, Gabor Nagy, María Victoria Nugnes, José Manuel Pérez-Cañadillas, Giulia Pesce, Roberta Pierattelli, Damiano Piovesan, Federica Quaglia, Sylvie Ricard-Blum, Paul Robustelli, Amin Sagar, Edoardo Salladini, Lucile Sénicourt, Nathalie Sibille, João M C Teixeira, Thomas E Tsangaris, Mihaly Varadi, Peter Tompa, Silvio C E Tosatto, Alexander Miguel Monzon
Nucleic Acids Research 52:D536-D544 (2024)10.1093/nar/gkad947
The disordered C-terminal tail of fungal LPMOs from phytopathogens mediates protein dimerization and impacts plant penetration
Ketty Tamburrini, Sayo Kodama, Sacha Grisel, Mireille Haon, Takumi Nishiuchi, Bastien Bissaro, Yasuyuki Kubo, Sonia Longhi, Jean-Guy Berrin
Proceedings of the National Academy of Sciences of the United States of America 121:e2319998121 (2024)10.1073/pnas.2319998121
Dissecting Henipavirus W proteins conformational and fibrillation properties: contribution of their N‐ and C‐terminal constituent domains
Giulia Pesce, Frank Gondelaud, Denis Ptchelkine, Christophe Bignon, Patrick Fourquet, Sonia Longhi
FEBS Journal Online ahead of print (2024)10.1111/febs.17239
DisProt in 2024: improving function annotation of intrinsically disordered proteins
Maria Cristina Aspromonte, Maria Victoria Nugnes, Federica Quaglia, Adel Bouharoua, Vasileios Sagris, Vasilis Promponas, Anastasia Chasapi, Erzsébet Fichó, Galo Balatti, Gustavo Parisi, Martín González Buitrón, Gabor Erdos, Matyas Pajkos, Zsuzsanna Dosztányi, Laszlo Dobson, Alessio Del Conte, Damiano Clementel, Edoardo Salladini, Emanuela Leonardi, Fatemeh Kordevani, Hamidreza Ghafouri, Luiggi Ku, Alexander Miguel Monzon, Carlo Ferrari, Zsófia Kálmán, Juliet Nilsson, Jaime Santos, Carlos Pintado-Grima, Salvador Ventura, Veronika Ács, Rita Pancsa, Mariane Goncalves Kulik, Miguel Andrade-Navarro, Pedro José Barbosa Pereira, Sonia Longhi, Philippe Le Mercier, Julian Bergier, Peter Tompa, Tamas Lazar, Silvio C E Tosatto, Damiano Piovesan
Nucleic Acids Research (2023)10.1093/nar/gkad928
Condensation of the N-terminal domain of human topoisomerase 1 is driven by electrostatic interactions and tuned by its charge distribution
Greta Bianchi, Marco Mangiagalli, Diletta Ami, Junaid Ahmed, Silvia Lombardi, Sonia Longhi, Antonino Natalello, Peter Tompa, Stefania Brocca
International Journal of Biological Macromolecules 254:127754 (2023)10.1016/j.ijbiomac.2023.127754
The Ustilago maydis AA10 LPMO is active on fungal cell wall chitin
Roseline Assiah Yao, Jean-Lou Reyre, Ketty Tamburrini, Mireille Haon, Olivier Tranquet, Akshay Nalubothula, Saumashish Mukherjee, Sophie Le Gall, Sacha Grisel, Sonia Longhi, Jogi Madhuprakash, Bastien Bissaro, Jean-Guy Berrin
Applied and Environmental Microbiology 89 (2023)10.1128/aem.00573-23
In Vivo Protein–Protein Binding Competition Assay Based on Split-GFP Reassembly: Proof of Concept
Christophe Bignon, Sonia Longhi
Biomolecules 13:354 (2023)10.3390/biom13020354
Liaisons dangereuses: Intrinsic Disorder in Cellular Proteins Recruited to Viral Infection-Related Biocondensates
Greta Bianchi, Stefania Brocca, Sonia Longhi, Vladimir N Uversky
International Journal of Molecular Sciences 24:2151 (2023)10.3390/ijms24032151
Molecular Determinants of Fibrillation in a Viral Amyloidogenic Domain from Combined Biochemical and Biophysical Studies
Juliet F Nilsson, Hakima Baroudi, Frank Gondelaud, Giulia Pesce, Christophe Bignon, Denis Ptchelkine, Joseph Chamieh, Hervé Cottet, Andrey V Kajava, Sonia Longhi
International Journal of Molecular Sciences 24:399 (2023)10.3390/ijms24010399
Per Aspera ad Chaos: Vladimir Uversky’s Odyssey through the Strange World of Intrinsically Disordered Proteins
Prakash Kulkarni, Stefania Brocca, A. Keith Dunker, Sonia Longhi
Biomolecules 13:1015 (2023)10.3390/biom13061015
Minimum information guidelines for experiments structurally characterizing intrinsically disordered protein regions
Bálint Mészáros, András Hatos, Nicolas Palopoli, Federica Quaglia, Edoardo Salladini, Kim van Roey, Haribabu Arthanari, Zsuzsanna Dosztányi, Isabella C Felli, Patrick D Fischer, Jeffrey C Hoch, Cy M Jeffries, Sonia Longhi, Emiliano Maiani, Sandra Orchard, Rita Pancsa, Elena Papaleo, Roberta Pierattelli, Damiano Piovesan, Iva Pritisanac, Luiggi Tenorio, Thibault Viennet, Peter Tompa, Wim Vranken, Silvio C E Tosatto, Norman E Davey
Nature Methods (2023)10.1038/s41592-023-01915-x
Viral amyloids: New opportunities for antiviral therapeutic strategies
Frank Gondelaud, Pierre-Yves Lozach, Sonia Longhi
Current Opinion in Structural Biology 83:102706 (2023)10.1016/j.sbi.2023.102706
Molecular determinants of fibrillation in a viral amyloidogenic domain from combined biochemical and biophysical studies
Juliet F Nilsson, Hakima Baroudi, Frank Gondelaud, Giulia Pesce, Christophe Bignon, Denis Ptchelkine, Joseph Chamieh, Hervé Cottet, Andrey V Kajava, Sonia Longhi
Split-GFP Reassembly Assay: Strengths and Caveats from a Multiparametric Analysis
Christophe Bignon, Antoine Gruet, Sonia Longhi
International Journal of Molecular Sciences 23:13167 (2022)10.3390/ijms232113167
Serum IgG levels to Epstein-Barr and measles viruses in patients with multiple sclerosis during natalizumab and interferon beta treatment
Linn Persson Berg, Marcus Eriksson, Sonia Longhi, Ingrid Kockum, Clemens Warnke, Elisabeth Thomsson, Malin Bäckström, Tomas Olsson, Anna Fogdell-Hahn, Tomas Bergström
BMJ Neurology Open 4:e000271 (2022)10.1136/bmjno-2022-000271
Predicting Protein Conformational Disorder and Disordered Binding Sites
Ketty Tamburrini, Giulia Pesce, Juliet Nilsson, Frank Gondelaud, Andrey Kajava, Jean-Guy Berrin, Sonia Longhi
2449:95-147 (2022)10.1007/978-1-0716-2095-3_4
Distribution of Charged Residues Affects the Average Size and Shape of Intrinsically Disordered Proteins
Greta Bianchi, Marco Mangiagalli, Alberto Barbiroli, Sonia Longhi, Rita Grandori, Carlo Santambrogio, Stefania Brocca
Biomolecules 12:561 (2022)10.3390/biom12040561
Association between common cardiovascular risk factors and clinical phenotype in patients with hypertrophic cardiomyopathy from the European Society of Cardiology (ESC) EurObservational Research Programme (EORP) Cardiomyopathy/Myocarditis registry
Luis Lopes, Maria-Angela Losi, Nabeel Sheikh, Cécile Laroche, Philippe Charron, Juan Gimeno, Juan Kaski, Aldo Maggioni, Luigi Tavazzi, Eloisa Arbustini, Dulce Brito, Jelena Celutkiene, Albert Hagege, Ales Linhart, Jens Mogensen, José Manuel Garcia-Pinilla, Tomas Ripoll-Vera, Hubert Seggewiss, Eduardo Villacorta, Alida Caforio, Perry Elliott, Christopher Peter Gale, Branko Beleslin, Andrzej Budaj, Ovidiu Chioncel, Nikolaos Dagres, Nicolas Danchin, David Erlinge, Jonathan Emberson, Michael Glikson, Alastair Gray, Meral Kayikcioglu, Aldo Maggioni, Klaudia Vivien Nagy, Aleksandr Nedoshivin, Anna-Sonia Petronio, Jolien Roo Hesselink, Lars Wallentin, Uwe Zeymer, Alida Caforio, Juan Ramon Gimeno Blanes, Philippe Charron, Perry Elliott, Juan Pablo Kaski, Aldo Maggioni, Luigi Tavazzi, Michal Tendera, S Komissarova, N Chakova, S Niyazova, A Linhart, P Kuchynka, T Palecek, J Podzimkova, M Fikrle, E Nemecek, H Bundgaard, J Tfelt-Hansen, J Theilade, J Thune, A Axelsson, J Mogensen, F Henriksen, T Hey, S Nielsen, L Videbaek, S Andreasen, H Arnsted, A Saad, M Ali, J Lommi, T Helio, M Nieminen, Olivier Dubourg, N Mansencal, M Arslan, V Siam Tsieu, T Damy, A Guellich, S Guendouz, C Tissot, A Lamine, S Rappeneau, A Hagege, M Desnos, A Bachet, M Hamzaoui, Philippe Charron, Richard Isnard, L Legrand, C Maupain, E Gandjbakhch, M Kerneis, J-F Pruny, A Bauer, B Pfeiffer, Stephan Felix, Marcus Dörr, S Kaczmarek, Kristin Lehnert, A-L Pedersen, D Beug, M Bruder, M Böhm, I Kindermann, Y Linicus, C Werner, B Neurath, M Schild-Ungerbuehler, H Seggewiss, B Pfeiffer, A Neugebauer, P Mckeown, A Muir, J Mcosker, T Jardine, G Divine, P Elliott, M Lorenzini, O Watkinson, E Wicks, H Iqbal, S Mohiddin, C O'Mahony, N Sekri, G Carr-White, T Bueser, R Rajani, L Clack, J Damm, S Jones, R Sanchez-Vidal, M Smith, T Walters, K Wilson, S Rosmini, A Anastasakis, K Ritsatos, V Vlagkouli, T Forster, R Sepp, J Borbas, V Nagy, A Tringer, K Kakonyi, L Szabo, M Maleki, F Noohi Bezanjani, A Amin, N Naderi, M Parsaee, S Taghavi, B Ghadrdoost, S Jafari, M Khoshavi, C Rapezzi, E Biagini, A Corsini, C Gagliardi, M Graziosi, S Longhi, A Milandri, L Ragni, S Palmieri, I Olivotto, A Arretini, G Castelli, F Cecchi, A Fornaro, B Tomberli, P Spirito, E Devoto, P Della Bella, G Maccabelli, S Sala, F Guarracini, G Peretto, M Russo, R Calabro, G Pacileo, G Limongelli, D Masarone, V Pazzanese, A Rea, M Rubino, S Tramonte, F Valente, M Caiazza, A Cirillo, G del Giorno, A Esposito, R Gravino, T Marrazzo, B Trimarco, M-A Losi, C Di Nardo, A Giamundo, F Musella, F Pacelli, A Scatteia, G Canciello, A Caforio, S Iliceto, C Calore, L Leoni, M Perazzolo Marra, I Rigato, G Tarantini, A Schiavo, M Testolina, Eloisa Arbustini, A Di Toro, L Giuliani, A Serio, F Fedele, A Frustaci, M Alfarano, C Chimenti, F Drago, A Baban, L Calò, C Lanzillo, A Martino, M Uguccioni, E Zachara, G Halasz, F Re, G Sinagra, C Carriere, M Merlo, F Ramani, A Kavoliuniene, A Krivickiene, E Tamuleviciute-Prasciene, M Viezelis, J Celutkiene, L Balkeviciene, M Laukyte, E Paleviciute, Y Pinto, A Wilde, Folkert Asselbergs, A Sammani, J van der Heijden, L van Laake, N de Jonge, R Hassink, J Kirkels, J Ajuluchukwu, A Olusegun-Joseph, E Ekure, K Mizia-Stec, M Tendera, A Czekaj, A Sikora-Puz, A Skoczynska, M Wybraniec, P Rubis, E Dziewiecka, S Wisniowska-Smialek, Zofia Bilinska, P Chmielewski, B Foss-Nieradko, E Michalak, M Stepien-Wojno, B Mazek, L Rocha Lopes, A Almeida, I Cruz, A Gomes, A Pereira, D Brito, H Madeira, A Francisco, M Menezes, O Moldovan, T Oliveira Guimaraes, D Silva, C Ginghina, R Jurcut, A Mursa, B Popescu, E Apetrei, S Militaru, I Mircea Coman, A Frigy, Z Fogarasi, I Kocsis, I Szabo, L Fehervari, I Nikitin, E Resnik, M Komissarova, V Lazarev, M Shebzukhova, D Ustyuzhanin, O Blagova, I Alieva, V Kulikova, Y Lutokhina, E Pavlenko, N Varionchik, A Ristic, P Seferovic, I Veljic, I Zivkovic, I Milinkovic, A Pavlovic, G Radovanovic, D Simeunovic, M Zdravkovic, M Aleksic, J Djokic, S Hinic, S Klasnja, K Mircetic, L Monserrat, X Fernandez, D Garcia-Giustiniani, J Larrañaga, M Ortiz-Genga, R Barriales-Villa, C Martinez-Veira, E Veira, A Cequier, J Salazar-Mendiguchia, N Manito, J Gonzalez, F Fernández-Avilés, C Medrano, R Yotti, S Cuenca, M Espinosa, I Mendez, E Zatarain, R Alvarez, P Garcia Pavia, A Briceno, M Cobo-Marcos, F Dominguez, E de Teresa Galvan, J Pinilla, N Abdeselam-Mohamed, M Lopez-Garrido, L Morcillo Hidalgo, M Ortega-Jimenez, a Robles Mezcua, A Guijarro-Contreras, D Gomez-Garcia, M Robles-Mezcua, J Blanes, F Castro, C Munoz Esparza, M Sabater Molina, M Sorli García, D Lopez Cuenca, Palma de Mallorca, T Ripoll-Vera, J Alvarez, J Nunez, Y Gomez, P Fernandez, E Villacorta, C Avila, L Bravo, E Diaz-Pelaez, M Gallego-Delgado, L Garcia-Cuenllas, B Plata, J Lopez-Haldon, M Pena Pena, E Perez, E Zorio, M Arnau, J Sanz, E Marques-Sule
European Heart Journal. Quality of Care and Clinical Outcomes 9:42-53 (2022)10.1093/ehjqcco/qcac006
Experimental Evidence of Intrinsic Disorder and Amyloid Formation by the Henipavirus W Proteins
Giulia Pesce, Frank Gondelaud, Denis Ptchelkine, Juliet F Nilsson, Christophe Bignon, Jérémy Cartalas, Patrick Fourquet, Sonia Longhi
International Journal of Molecular Sciences 23:923 (2022)10.3390/ijms23020923
DisProt in 2022: improved quality and accessibility of protein intrinsic disorder annotation
Federica Quaglia, Bálint Mészáros, Edoardo Salladini, András Hatos, Rita Pancsa, Lucía B Chemes, Mátyás Pajkos, Tamas Lazar, Samuel Peña-Díaz, Jaime Santos, Veronika Ács, Nazanin Farahi, Erzsébet Fichó, Maria Cristina Aspromonte, Claudio Bassot, Anastasia Chasapi, Norman E Davey, Radoslav Davidović, Laszlo Dobson, Arne Elofsson, Gábor Erdős, Pascale Gaudet, Michelle Giglio, Juliana Glavina, Javier Iserte, Valentín Iglesias, Zsófia Kálmán, Matteo Lambrughi, Emanuela Leonardi, Sonia Longhi, Sandra Macedo-Ribeiro, Emiliano Maiani, Julia Marchetti, Cristina Marino-Buslje, Attila Mészáros, Alexander Miguel Monzon, Giovanni Minervini, Suvarna Nadendla, Juliet F Nilsson, Marian Novotný, Christos A Ouzounis, Nicolás Palopoli, Elena Papaleo, Pedro José Barbosa Pereira, Gabriele Pozzati, Vasilis J Promponas, Jordi Pujols, Alma Carolina Sanchez Rocha, Martin Salas, Luciana Rodriguez Sawicki, Eva Schad, Aditi Shenoy, Tamás Szaniszló, Konstantinos D Tsirigos, Nevena Veljkovic, Gustavo Parisi, Salvador Ventura, Zsuzsanna Dosztányi, Peter Tompa, Silvio C E Tosatto, Damiano Piovesan
Nucleic Acids Research 50:D480-D487 (2022)10.1093/nar/gkab1082
Functional benefit of structural disorder for the replication of measles, Nipah and Hendra viruses
Frank Gondelaud, Giulia Pesce, Juliet F. Nilsson, Christophe Bignon, Denis Ptchelkine, Denis Gerlier, Cyrille Mathieu, Sonia Longhi
Essays in Biochemistry 66:915-934 (2022)10.1042/EBC20220045
Identification of a Region in the Common Amino-terminal Domain of Hendra Virus P, V, and W Proteins Responsible for Phase Transition and Amyloid Formation
Edoardo Salladini, Frank Gondelaud, Juliet Nilsson, Giulia Pesce, Christophe Bignon, Maria Murrali, Roxane Fabre, Roberta Pierattelli, Andrey Kajava, Branka Horvat, Denis Gerlier, Cyrille Mathieu, Sonia Longhi
Biomolecules 11:1324 (2021)10.3390/biom11091324
Insights into the evolutionary forces that shape the codon usage in the viral genome segments encoding intrinsically disordered protein regions
Naveen Kumar, Rahul Kaushik, Chandana Tennakoon, Vladimir N Uversky, Sonia Longhi, Kam y J Zhang, Sandeep Bhatia
Briefings in Bioinformatics 22 (2021)10.1093/bib/bbab145
Structural and Functional Characterization of the ABA-Water Deficit Stress Domain from Wheat and Barley: An Intrinsically Disordered Domain behind the Versatile Functions of the Plant Abscissic Acid, Stress and Ripening Protein Family
Ines Yacoubi, Karama Hamdi, Patrick Fourquet, Christophe Bignon, Sonia Longhi
International Journal of Molecular Sciences 22:2314 (2021)10.3390/ijms22052314
Structural and dynamics analysis of intrinsically disordered proteins by high-speed atomic force microscopy
Noriyuki Kodera, Daisuke Noshiro, Sujit K Dora, Tetsuya Mori, Johnny Habchi, David Blocquel, Antoine Gruet, Marion Dosnon, Edoardo Salladini, Christophe Bignon, Yuko Fujioka, Takashi Oda, Nobuo N Noda, Mamoru Sato, Marina Lotti, Mineyuki Mizuguchi, Sonia Longhi, Toshio Ando
Nature Nanotechnology 16:181-189 (2021)10.1038/s41565-020-00798-9
PED in 2021: a major update of the protein ensemble database for intrinsically disordered proteins
Tamas Lazar, Elizabeth Martínez-Pérez, Federica Quaglia, András Hatos, Lucía Chemes, Javier Iserte, Nicolás Méndez, Nicolás Garrone, Tadeo Saldaño, Julia Marchetti, Ana Julia Velez Rueda, Pau Bernadó, Martin Blackledge, Tiago Cordeiro, Eric Fagerberg, Julie Forman-Kay, Maria Fornasari, Toby Gibson, Gregory-Neal Gomes, Claudiu Gradinaru, Teresa Head-Gordon, Malene Ringkjøbing Jensen, Edward Lemke, Sonia Longhi, Cristina Marino-Buslje, Giovanni Minervini, Tanja Mittag, Alexander Miguel Monzon, Rohit Pappu, Gustavo Parisi, Sylvie Ricard-Blum, Kiersten Ruff, Edoardo Salladini, Marie Skepö, Dmitri Svergun, Sylvain Vallet, Mihaly Varadi, Peter Tompa, Silvio Tosatto, Damiano Piovesan
Nucleic Acids Research 49:D404-D411 (2021)10.1093/nar/gkaa1021
Bioinformatic Analysis of Lytic Polysaccharide Monooxygenases Reveals the Pan-Families Occurrence of Intrinsically Disordered C-Terminal Extensions
Ketty C Tamburrini, Nicolas Terrapon, Vincent Lombard, Bastien Bissaro, Sonia Longhi, Jean-Guy Berrin
Biomolecules 11:1632 (2021)10.3390/biom11111632
Comprehensive Intrinsic Disorder Analysis of 6108 Viral Proteomes: From the Extent of Intrinsic Disorder Penetrance to Functional Annotation of Disordered Viral Proteins
Naveen Kumar, Rahul Kaushik, Chandana Tennakoon, Vladimir N Uversky, Sonia Longhi, Kam y J Zhang, Sandeep Bhatia
Journal of Proteome Research 20:2704-2713 (2021)10.1021/acs.jproteome.1c00011
Extended disorder at the cell surface: The conformational landscape of the ectodomains of syndecans
Frank Gondelaud, Mathilde Bouakil, Aurélien Le Fèvre, Adriana Erica Miele, Fabien Chirot, Bertrand Duclos, Adam Liwo, Sylvie Ricard-Blum
Matrix Biology Plus 12:100081 (2021)10.1016/j.mbplus.2021.100081
PED in 2021: a major update of the protein ensembledatabase for intrinsically disordered proteins
Tamas Lazar, Elizabeth Martínez-Pérez, Federica Quaglia, András Hatos, Lucía B Chemes, Javier A Iserte, Nicolás A Méndez, Nicolás A Garrone, Tadeo E Saldaño, Julia Marchetti, Ana Julia Velez Rueda, Pau Bernadó, Martin Blackledge, Tiago N Cordeiro, Eric Fagerberg, Julie D Forman-Kay, Maria S Fornasari, Toby J Gibson, Gregory-Neal W Gomes, Claudiu C Gradinaru, Teresa Head-Gordon, Malene Ringkjøbing Jensen, Edward A Lemke, Sonia Longhi, Cristina Marino-Buslje, Giovanni Minervini, Tanja Mittag, Alexander Miguel Monzon, Rohit V Pappu, Gustavo Parisi, Sylvie Ricard-Blum, Kiersten M Ruff, Edoardo Salladini, Marie Skepö, Dmitri Svergun, Sylvain D Vallet, Mihaly Varadi, Peter Tompa, Silvio C E Tosatto, Damiano Piovesan, Rodolfo A Ugalde
Nucleic Acids Research (2020)10.1093/nar/gkaa1021
Predicting substitutions to modulate disorder and stability in coiled-coils
Yasaman Karami, Paul Saighi, Rémy Vanderhaegen, Denis Gerlier, Sonia Longhi, Elodie Laine, Alessandra Carbone
BMC Bioinformatics 21:573 (2020)10.1186/s12859-020-03867-x
Liquid-Liquid Phase Separation by Intrinsically Disordered Protein Regions of Viruses: Roles in Viral Life Cycle and Control of Virus-Host Interactions
Stefania Brocca, Rita Grandori, Sonia Longhi, Vladimir Uversky
International Journal of Molecular Sciences (2020)10.3390/ijms21239045
Ensemble description of the intrinsically disordered N-terminal domain of the Nipah virus P/V protein from combined NMR and SAXS
Marco Schiavina, Edoardo Salladini, Maria Grazia Murrali, Giancarlo Tria, Isabella Felli, Roberta Pierattelli, Sonia Longhi
Scientific Reports 10 (2020)10.1038/s41598-020-76522-3
Caractérisation d’une famille de protéines intrinsèquement désordonnées, les syndécans
Frank Gondelaud
(2020)
Relevance of Electrostatic Charges in Compactness, Aggregation, and Phase Separation of Intrinsically Disordered Proteins
Greta Bianchi, Sonia Longhi, Rita Grandori, Stefania Brocca
International Journal of Molecular Sciences 21:6208 (2020)10.3390/ijms21176208
The endosomal lipid bis(monoacylglycero) phosphate as a potential key player in the mechanism of action of chloroquine against SARS-COV-2 and other enveloped viruses hijacking the endocytic pathway
Frédéric Carrière, Sonia Longhi, Michel Record
Biochimie 179:237-246 (2020)10.1016/j.biochi.2020.05.013
Sialic acids rather than glycosaminoglycans affect normal and sickle red blood cell rheology by binding to four major sites on fibrinogen
Frank Gondelaud, Philippe Connes, Elie Nader, Céline Renoux, Romain Fort, Alexandra Gauthier, Philippe Joly, Sylvie Ricard‐blum
American Journal of Hematology (2020)10.1002/ajh.25718
Phase transition and amyloid formation by a viral protein as an additional molecular mechanism of virus-induced cell toxicity
Edoardo Salladini, Claire Debarnot, Vincent Delauzun, Maria Grazia Murrali, Priscila Sutto-Ortiz, Silvia Spinelli, Roberta Pierattelli, Christophe Bignon, Sonia Longhi
(2019)
Conformational footprinting of proteins using a combination of top- down electron transfer dissociation and ion mobility
Albert Konijnenberg, Jinyu Li, Johny Habchi, Marion Dosnon, Giulia Rossetti, Rita Grandori, Sonia Longhi, Paolo Carloni, Frank Sobott
(2019)10.1101/283796
Probing the dynamic properties of two sites simultaneously in a protein–protein interaction process: a SDSL-EPR study
N. Le Breton, S. Longhi, A. Rockenbauer, B. Guigliarelli, S. Marque, V. Belle, M. Martinho
Physical Chemistry Chemical Physics 21:22584-22588 (2019)10.1039/c9cp04660g
Binding induced folding: Lessons from the kinetics of interaction between NTAIL and XD
Angelo Toto, Francesca Troilo, Lorenzo Visconti, Francesca Malagrinò, Christophe Bignon, Sonia Longhi, Stefano Gianni
Archives of Biochemistry and Biophysics 671:255-261 (2019)10.1016/j.abb.2019.07.011
An arsenal of methods for the experimental characterization of intrinsically disordered proteins – How to choose and combine them?
Antoine Schramm, Christophe Bignon, Stefania Brocca, Rita Grandori, Carlo Santambrogio, Sonia Longhi
Archives of Biochemistry and Biophysics 108055 (2019)10.1016/j.abb.2019.07.020
Structures and interactions of syndecans
Frank Gondelaud, Sylvie Ricard-Blum
FEBS Journal 286:2994-3007 (2019)10.1111/febs.14828
Understanding Intramolecular Crosstalk in an Intrinsically Disordered Protein
Francesca Troilo, Daniela Bonetti, Christophe Bignon, Sonia Longhi, Stefano Gianni
ACS Chemical Biology 14:337-341 (2019)10.1021/acschembio.8b01055
Modulation of Measles Virus NTAIL Interactions through Fuzziness and Sequence Features of Disordered Binding Sites
Christophe Bignon, Francesca Troilo, Stefano Gianni, Sonia Longhi
Biomolecules 9:8 (2019)10.3390/biom9010008
Regulation of measles virus gene expression by P protein coiled-coil properties
Louis-Marie Bloyet, Antoine Schramm, Carine Lazert, Bertrand Raynal, Maggy Hologne, Olivier Walker, Sonia Longhi, Denis Gerlier
Science Advances 5:eaaw3702 (2019)10.1126/sciadv.aaw3702
Conformational response to charge clustering in synthetic intrinsically disordered proteins
Giulia Tedeschi, Edoardo Salladini, Carlo Santambrogio, Rita Grandori, Sonia Longhi, Stefania Brocca
Biochimica et Biophysica Acta (BBA) - General Subjects 1862:2204-2214 (2018)10.1016/j.bbagen.2018.07.011
Folding Mechanism of the SH3 Domain from Grb2
Francesca Troilo, Daniela Bonetti, Carlo Camilloni, Angelo Toto, Sonia Longhi, Maurizio Brunori, Stefano Gianni
Journal of Physical Chemistry B 122:11166-11173 (2018)10.1021/acs.jpcb.8b06320
Exploration of nucleoprotein α-MoRE and XD interactions of Nipah and Hendra viruses
Xu Xu, Wenting Chu, Xiakun Chu, Liufang Xu, Sonia Longhi, Jin Wang, Xu Shang
Journal of Molecular Modeling 24:113 (2018)10.1007/s00894-018-3643-6
How Robust Is the Mechanism of Folding-Upon-Binding for an Intrinsically Disordered Protein?
Daniela Bonetti, Francesca Troilo, Maurizio Brunori, Sonia Longhi, Stefano Gianni
Biophysical Journal 114:1889-1894 (2018)10.1016/j.bpj.2018.03.017
InSiDDe: A Server for Designing Artificial Disordered Proteins
Antoine Schramm, Philippe Lieutaud, Stefano Gianni, Sonia Longhi, Christophe Bignon
International Journal of Molecular Sciences 19:e91 (2018)10.3390/ijms19010091
Experimental Characterization of Fuzzy Protein Assemblies: Interactions of Paramyxoviral NTAIL Domains With Their Functional Partners
Francesca Troilo, Christophe Bignon, Stefano Gianni, Monika Fuxreiter, Sonia Longhi
611:137-192 (2018)10.1016/bs.mie.2018.08.006
Partner-Mediated Polymorphism of an Intrinsically Disordered Protein
Christophe Bignon, Francesca Troilo, Stefano Gianni, Sonia Longhi
Journal of Molecular Biology (2017)10.1016/j.jmb.2017.11.012
Comment la ré-initiation de la transcription est-elle gouvernée chez le virus de la rougeole ?
Louis-Marie Bloyet, Philippe Roche, Sonia Longhi, Denis Gerlier
Médecine/Sciences 33:843-845 (2017)10.1051/medsci/20173310010
How order and disorder within paramyxoviral nucleoproteins and phosphoproteins orchestrate the molecular interplay of transcription and replication
Sonia Longhi, Louis-Marie Bloyet, Stefano Gianni, Denis Gerlier
Cellular and Molecular Life Sciences 74:3091-3118 (2017)10.1007/s00018-017-2556-3
Analyzing the Folding and Binding Steps of an Intrinsically Disordered Protein by Protein Engineering
Daniela Bonetti, Francesca Troilo, Angelo Toto, Maurizio Brunori, Sonia Longhi, Stefano Gianni
Biochemistry 56:3780-3786 (2017)10.1021/acs.biochem.7b00350
Simultaneous quantification of protein order and disorder using NMR spectroscopy
Pietro Sormanni, Damiano Piovesan, Gabriella T Heller, Massimiliano Bonomi, Predrag Kukic, Carlo Camilloni, Monika Fuxreiter, Zsuzsanna Dosztanyi, Rohit Pappu, M Madan Babu, Sonia Longhi, Peter Tompa, A Keith Dunker, Vladimir N Uversky, Silvio C E Tosatto, Michele Vendruscolo
Nature Chemical Biology (2017)10.1038/nchembio.2331
Simultaneous quantification of protein order and disorder
Pietro Sormanni, Damiano Piovesan, Gabriella Heller, Massimiliano Bonomi, Predrag Kukic, Carlo Camilloni, Monika Fuxreiter, Zsuzsanna Dosztanyi, Rohit Pappu, M Madan Babu, Sonia Longhi, Peter Tompa, a Keith Dunker, Vladimir Uversky, Silvio Tosatto, Michele Vendruscolo
Nature Chemical Biology 13:339-342 (2017)10.1038/nchembio.2331
The Folding Pathway of the KIX Domain
Francesca Troilo, Daniela Bonetti, Angelo Toto, Lorenzo Visconti, Maurizio Brunori, Sonia Longhi, Stefano Gianni
ACS Chemical Biology 12:1683-1690 (2017)10.1021/acschembio.7b00289
Probing Conformational Changes and Interfacial Recognition Site of Lipases With Surfactants and Inhibitors
Eduardo Mateos Diaz, S, Amara, A Roussel, Sonia Longhi, Christian Cambillau, Frédéric Carrière
Methods in Enzymology 279-307 (2017)
The Henipavirus V protein is a prevalently unfolded protein with a zinc-finger domain involved in binding to DDB1
Sonia Longhi, Vincent Delauzun, Edoardo Salladini
Molecular BioSystems 13:2254-2267 (2017)10.1039/c7mb00488e
Interfacial Properties of N TAIL , an Intrinsically Disordered Protein
Anaïs Benarouche, Johnny Habchi, Alain Cagna, Ofelia Maniti, Agnès P Girard-Egrot, Jean-François Cavalier, Sonia Longhi, Frédéric Carriere
Biophysical Journal 113:2723-2735 (2017)10.1016/j.bpj.2017.10.010
DisProt 7.0: a major update of the database of disordered proteins
Damiano Piovesan, Francesco Tabaro, Ivan Mičetić, Marco Necci, Federica Quaglia, Christopher J. Oldfield, Maria Cristina Aspromonte, Norman E. Davey, Radoslav Davidović, Zsuzsanna Dosztányi, Arne Elofsson, Alessandra Gasparini, András Hatos, Andrey V. Kajava, Lajos Kalmar, Emanuela Leonardi, Tamas Lazar, Sandra Macedo-Ribeiro, Mauricio Macossay-Castillo, Attila Meszaros, Giovanni Minervini, Nikoletta Murvai, Jordi Pujols, Daniel Barry Roche, Edoardo Salladini, Eva Schad, Antoine Schramm, Beata Szabo, Agnes Tantos, Fiorella Tonello, Konstantinos D. Tsirigos, Nevena Veljković, Salvador Ventura, Wim Vranken, Per Warholm, Vladimir N. Uversky, A.~keith Dunker, Sonia Longhi, Peter Tompa, Silvio C.E. Tosatto
Nucleic Acids Research 45:D219-D227 (2017)10.1093/nar/gkw1056
Structural disorder and induced folding within two cereal, ABA stress and ripening (ASR) proteins
Karama Hamdi, Edoardo Salladini, Darragh O'Brien, Sebastien Brier, Alexandre Chenal, Ines Yacoubi, Sonia Longhi
Scientific Reports 7:15544 (2017)10.1038/s41598-017-15299-4
Modulation of Re-initiation of Measles Virus Transcription at Intergenic Regions by PXD to NTAIL Binding Strength
Louis-Marie Bloyet, Joanna Brunel, Marion Dosnon, Véronique Hamon, Jenny Erales, Antoine Gruet, Carine Lazert, Christophe Bignon, Philippe Roche, Sonia Longhi, Denis Gerlier
PLoS Pathogens 12:e1006058 (2016)10.1371/journal.ppat.1006058
Identification and Structural Characterization of an Intermediate in the Folding of the Measles Virus X Domain
Daniela Bonetti, Carlo Camilloni, Lorenzo Visconti, Sonia Longhi, Maurizio Brunori, Michele Vendruscolo, Stefano Gianni
Journal of Biological Chemistry 291:10886+ (2016)10.1074/jbc.M116.721126
Predicting Conformational Disorder
Philippe Lieutaud, François Ferron, Sonia Longhi
1415:265-299 (2016)10.1007/978-1-4939-3572-7_14
Fuzzy regions in an intrinsically disordered protein impair protein-protein interactions
Antoine Gruet, Marion Dosnon, David Blocquel, Joanna Brunel, Denis Gerlier, Rahul K. Das, Daniela Bonetti, Stefano Gianni, Monika Fuxreiter, Sonia Longhi, Christophe Bignon
FEBS Journal 283:576-594 (2016)10.1111/febs.13631
How disordered is my protein and what is its disorder for? A guide through the “dark side” of the protein universe
Philippe Lieutaud, François Ferron, Alexey V Uversky, Lukasz Kurgan, Vladimir N Uversky, Sonia Longhi
Intrinsically Disordered Proteins 4:e1259708 (2016)10.1080/21690707.2016.1259708
Structural disorder within paramyxoviral nucleoproteins
Sonia Longhi
FEBS Letters 589:2649-2659 (2015)10.1016/j.febslet.2015.05.055
Insights into the Hendra virus N-TAIL-XD complex: Evidence for a parallel organization of the helical MoRE at the XD surface stabilized by a combination of hydrophobic and polar interactions
Jenny Erales, Matilde Beltrandi, Jennifer Roche, Maria Mate, Sonia Longhi
Biochimica et Biophysica Acta Proteins and Proteomics 1854:1038-1053 (2015)10.1016/j.bbapap.2015.04.031
Structural Disorder within Paramyxoviral Nucleoproteins and Phosphoproteins in Their Free and Bound Forms: From Predictions to Experimental Assessment
Johnny Habchi, Sonia Longhi
International Journal of Molecular Sciences 16:15688-15726 (2015)10.3390/ijms160715688
Mechanisms of partner recognition by intrinsically disordered proteins
M. Dosnon, A. Gruet, D. Blocquel, J. Erales, A. Morrone, D. Bonetti, M. Fuxreiter, S. Gianni, C. Bignon, S. Longhi
European Biophysics Journal 44:S229 (2015)
Insights into the coiled-coil organization of the Hendra virus phosphoprotein from combined biochemical and SAXS studies
Matilde Beltrandi, David Blocquel, Jenny Erales, Pascale Barbier, Andrea Cavalli, Sonia Longhi
Virology 477:42-55 (2015)10.1016/j.virol.2014.12.029
Demonstration of a Folding after Binding Mechanism in the Recognition between the Measles Virus N-TAIL and X Domains
Marion Dosnon, Daniela Bonetti, Angela Morrone, Jenny Erales, Eva Di Silvio, Sonia Longhi, Stefano Gianni
ACS Chemical Biology 10:795-802 (2015)10.1021/cb5008579
Molecular Basis for Structural Heterogeneity of an Intrinsically Disordered Protein Bound to a Partner by Combined ESI-IM-MS and Modeling
Annalisa d'Urzo, Albert Konijnenberg, Giulia Rossetti, Johnny Habchi, Jinyu Li, Paolo Carloni, Frank Sobott, Sonia Longhi, Rita Grandori
Journal of The American Society for Mass Spectrometry 26:472-481 (2015)10.1007/s13361-014-1048-z
Dynamics of the Intrinsically Disordered C-Terminal Domain of the Nipah Virus Nucleoprotein and Interaction with the X Domain of the Phosphoprotein as Unveiled by NMR Spectroscopy
Lorenzo Baronti, Jenny Erales, Johnny Habchi, Isabella C. Felli, Roberta Pierattelli, Sonia Longhi
ChemBioChem 16:268-276 (2015)10.1002/cbic.201402534
Developing a Protocol for Ensemble and Vibrational Probe-Containing Molecular Dynamics Simulations of the Nipah Ntail-XD Complex
Shana R. Burstein, Rebecca B. Wai, Sara K. Hess, Casey H. Londergan, Jenny Erales, Sonia Longhi
Biophysical Journal 108:227A (2015)
Assessing Binding Perturbation due to Artificial Vibrational Probe Groups in the Nucleoprotein-Phosphoprotein Complex of the Nipah Virus
Rebecca B. Wai, Shana R. Burstein, Sara K. Hess, Jenny Erales, Sonia Longhi, Casey H. Londergan
Biophysical Journal 108:389A (2015)
Order and Disorder in the Replicative Complex of Paramyxoviruses
Marion Dosnon, Christophe Bignon, Sonia Longhi, Jenny Erales, David Blocquel, Johnny Habchi, Matilde Beltrandi, Antoine Gruet
870:351-381 (2015)10.1007/978-3-319-20164-1_12
Sequence of events in measles virus replication: role of phosphoprotein-nucleocapsid interactions
Joanna Brunel, Damien Chopy, Marion Dosnon, Louis-Marie Bloyet, Patricia Devaux, Erica Urzua, Roberto Cattaneo, Sonia Longhi, Denis Gerlier
Journal of Virology 88:10851-10863 (2014)10.1128/JVI.00664-14
Introducing Protein Intrinsic Disorder
Johnny Habchi, Peter Tompa, Sonia Longhi, Vladimir N Uversky
Chemical Reviews 114:6561-6588 (2014)10.1021/cr400514h
Experimental data on the interactions between a viral pre-molten globule (VPg) and an eukaryotic translation initiation factor (eIF4E)
Thierry Michon, Amandine Barra, Justine Charon, Eugénie Hébrard, Sonia Longhi, Benoît Moury, Jocelyne J. Walter
(2014)
An attempt to experimentally assess the contribution of intrinsic disorder to the virus adaptation
Justine Charon, Amandine Barra, Eugénie Hébrard, Sonia Longhi, Benoît Moury, Jocelyne J. Walter, Thierry Michon
(2014)
Sequence of Events in Measles Virus Replication: Role of Phosphoprotein-Nucleocapsid Interactions
Joanna Brunel, Damien Chopy, Marion Dosnon, Louis-Marie Bloyet, Patricia Devaux, Erica Urzua, Roberto Cattaneo, Sonia Longhi, Denis Gerlier
Journal of Virology 88:10851-10863 (2014)10.1128/JVI.00664-14
Diversification of EPR signatures in site directed spin labeling using a beta-phosphorylated nitroxide
Nolwenn Le Breton, Marlène Martinho, Kuanysh Kabytaev, Jérémie Topin, Elisabetta Mileo, David Blocquel, Johnny Habchi, Sonia Longhi, Antal Rockenbauer, Jérôme Golebiowski, Bruno Guigliarelli, Sylvain R.A. Marque, Valérie Belle
Physical Chemistry Chemical Physics 16:4202-4209 (2014)10.1039/c3cp54816c
Atomic resolution description of the interaction between the nucleoprotein and phosphoprotein of Hendra virus.
Guillaume Communie, Johnny Habchi, Filip Yabukarski, David Blocquel, Robert Schneider, Nicolas Tarbouriech, Nicolas Papageorgiou, Rob W H Ruigrok, Marc Jamin, Malene Ringkjøbing Jensen, Sonia Longhi, Martin Blackledge
PLoS Pathogens 9:e1003631 (2013)
Dissecting Partner Recognition by an Intrinsically Disordered Protein Using Descriptive Random Mutagenesis
Antoine Gruet, Marion Dosnon, Andrea Vassena, Vincent Lombard, Denis Gerlier, Christophe Bignon, Sonia Longhi
Journal of Molecular Biology 425:3495-3509 (2013)10.1016/j.jmb.2013.06.025
Assessing induced folding within the intrinsically disordered C-terminal domain of the Henipavirus nucleoproteins by site directed spin labeling EPR spectroscopy
Marlène Martinho, Johnny Habchi, Zeina El Habre, Léo Nesme, Bruno Guigliarelli, Valérie Belle, Sonia Longhi
Journal of Biomolecular Structure and Dynamics 31:453-471 (2013)10.1080/07391102.2012.706068
Solution conformational features and interfacial properties of an intrinsically disordered peptide coupled to alkyl chains: a new class of peptide amphiphiles
Antonella Accardo, Marilisa Leone, Diego Tesauro, Rosa Aufiero, Anaïs Bénarouche, Jean-François Cavalier, Sonia Longhi, Frederic Carriere, Filomena Rossi
Molecular BioSystems 9:1401 (2013)10.1039/c3mb25507g
Plasticity in Structural and Functional Interactions between the Phosphoprotein and Nucleoprotein of Measles Virus
Yaoling Shu, Johnny Habchi, Stéphanie Costanzo, Andre Padilla, Joanna Brunel, Denis Gerlier, Michael Oglesbee, Sonia Longhi
Journal of Biological Chemistry 287:11951-11967 (2012)10.1074/jbc.M111.333088
Transcription et réplication des Mononegavirales : une machine moléculaire originale
David Blocquel, Jean-Marie Bourhis, Jean Francois J. F. Eleouet, Denis Gerlier, Johnny Habchi, Marc Jamin, Sonia Longhi, Filip Yabukarski
Virologie 16:225-257 (2012)10.1684/vir.2012.0458
Compaction and binding properties of the intrinsically disordered C-terminal domain of Henipavirus nucleoprotein as unveiled by deletion studies
David Blocquel, Johnny Habchi, Antoine Gruet, Stéphanie Blangy, Sonia Longhi
Molecular BioSystems 8:392-410 (2012)10.1039/c1mb05401e
Dividing To Unveil Protein Microheterogeneities: Traveling Wave Ion Mobility Study.
Frédéric Halgand, J. Habchi, Laetitia Cravello, Marlène Martinho, Bruno Guigliarelli, Sonia Longhi
Analytical Chemistry 19:7306-7315 (2011)
Probing structural transitions in both structured and disordered proteins using site-directed spin-labeling EPR spectroscopy
Sonia Longhi, Valérie Belle, André Fournel, Bruno Guigliarelli, Frédéric Carrière
Journal of Peptide Science 17:315-328 (2011)10.1002/psc.1344
Characterization of the Interactions between the Nucleoprotein and the Phosphoprotein of Henipavirus
Johnny Habchi, Stéphanie Blangy, Laurent Mamelli, Malene Ringkjøbing Jensen, Martin Blackledge, Herve Darbon, Michael Oglesbee, Yaoling Shu, Sonia Longhi
Journal of Biological Chemistry 286:13583-13602 (2011)10.1074/jbc.M111.219857
Conformational analysis of the partially disordered measles virus NTAIL-XD complex by SDSL EPR spectroscopy
A. Kavalenka, I. Urbancic, Valérie Belle, S. Rouger, S. Costanzo, S. Kure, André Fournel, Sonia Longhi, Bruno Guigliarelli, J. Strancar
Biophysical Journal 6:1055-1064 (2010)
Interaction between the C-terminal domains of N and P proteins of measles virus investigated by NMR.
Cedric Bernard, Stéphane Gely, Jean-Marie Bourhis, Xavier Morelli, Sonia Longhi, Hervé Darbon
FEBS Letters 583:1084-9 (2009)10.1016/j.febslet.2009.03.004
Modular Organization of Rabies Virus Phosphoprotein
Francine C.A. Gerard, Euripedes de Almeida Ribeiro Jr, Cedric Leyrat, Ivan Ivanov, Danielle Blondel, Sonia Longhi, Rob W.H. Ruigrok, Marc Jamin
Journal of Molecular Biology 388:978-996 (2009)10.1016/j.jmb.2009.03.061
The interaction between the measles virus nucleoprotein and the Interferon Regulator Factor 3 relies on a specific cellular environment
Matteo Colombo, Jean-Marie Bourhis, Célia Chamontin, Carine Soriano, Stéphanie Villet, Stéphanie Costanzo, Marie M. Couturier, Valérie Belle, André Fournel, Hervé Darbon, Denis Gerlier, Sonia Longhi
Virology Journal 6:59 (2009)10.1186/1743-422X-6-59
Intrinsic disorder in Viral Proteins Genome-Linked: experimental and predictive analyses
Eugénie Hébrard, Yannick Bessin, Thierry Michon, Sonia Longhi, Vladimir N Uversky, François Delalande, Alain van Dorsselaer, Pedro Romero, Jocelyne Walter, Nathalie Declerck, Denis Fargette
Virology Journal 6:23 (2009)10.1186/1743-422X-6-23
Rules Governing Selective Protein Carbonylation
Etienne Maisonneuve, Adrien Ducret, Pierre Khoueiry, Sabrina Lignon, Sonia Longhi, Emmanuel Talla, Sam Dukan
PLoS ONE 4:e7269 (2009)10.1371/journal.pone.0007269
Cytosolic 5'-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response.
Sébastien Plumet, Florence Herschke, Jean-Marie Bourhis, Hélène Valentin, Sonia Longhi, Denis Gerlier
PLoS ONE 2:e279 (2007)10.1371/journal.pone.0000279
SPINE bioinformatics and data-management aspects of high-throughput structural biology.
S. Albeck, Pedro Maria Alzari, C. Andreini, L. Banci, I. M. Berry, I. Bertini, C. Cambillau, B. Canard, L. Carter, S. X. Cohen, J. M. Diprose, O. Dym, R. M. Esnouf, C. Felder, F. Ferron, F. Guillemot, R. Hamer, M. Ben Jelloul, R. A. Laskowski, T. Laurent, S. Longhi, R. Lopez, C. Luchinat, H. Malet, T. Mochel, R. J. Morris, L. Moulinier, T. Oinn, A. Pajon, Y. Peleg, A. Perrakis, O. Poch, J. Prilusky, A. Rachedi, R. Ripp, A. Rosato, I. Silman, D. I. Stuart, J. L. Sussman, J. C. Thierry, J. D. Thompson, J. M. Thornton, T. Unger, B. Vaughan, W. Vranken, J. D. Watson, G. Whamond, K. Henrick
Acta crystallographica Section D : Structural biology [1993-...] 62:1184-95 (2006)10.1107/S090744490602991X
VaZyMolO: a tool to define and classify modularity in viral proteins
François Ferron, Corinne Rancurel, Sonia Longhi, Christian Cambillau, Bernard Henrissat, Bruno Canard
Journal of General Virology 86:743-749 (2005)10.1099/vir.0.80590-0
The severe acute respiratory syndrome-coronavirus replicative protein nsp9 is a single-stranded RNA-binding subunit unique in the RNA virus world
Marie-Pierre Egloff, Francois Ferron, Valérie Campanacci, Sonia Longhi, Corinne Rancurel, Hélène Dutartre, Eric J Snijder, Alexander Gorbalenya, Christian Cambillau, Bruno Canard
Proceedings of the National Academy of Sciences of the United States of America 101:3792-3796 (2004)10.1073/pnas.0307877101
Structural genomics of the SARS coronavirus: cloning, expression, crystallization and preliminary crystallographic study of the Nsp9 protein
Valérie Campanacci, Marie-Pierre Egloff, Sonia Longhi, François Ferron, Corinne Rancurel, Aurelia Salomoni, Cécile Durousseau, Fabienne Tocque, Nicolas Brémond, Jessika Dobbe, Eric Snijder, Bruno Canard, Christian Cambillau
Acta Crystallographica Section D: Biological Crystallography 59:1628-1631 (2003)10.1107/S0907444903016779
Molecular cloning of the cDNA encoding laccase from Pycnoporus cinnabarinus I-937 and expression in Pichia pastoris
L. Otterbein, Eric Record, S. Longhi, M. Asther, Serge Moukha
European Journal of Biochemistry 267:1619-1625 (2000)
Recombinant pheromone binding protein 1 from Mamestra brassicae (MbraPBP1). Functional and structural characterization
V. Campanacci, S. Longhi, Patricia Nagnan-Le Meillour, C. Cambillau, M. Tegoni
European Journal of Biochemistry 264:707-716 (1999)
Exploring hydrophobic sites in proteins with xenon or krypton
Thierry Prangé, Marc Schiltz, Lucile Pernot, Nathalie Colloc'H, Sonia Longhi, William Bourguet, Roger Fourme
Proteins: Structure, Function, and Genetics 30:61-73 (1998)10.1002/(SICI)1097-0134(19980101)30:1<61::AID-PROT6>3.0.CO;2-N
Molecular cloning and bacterial expression of a general odorant-binding protein from the cabbage armyworm Mamestra brassicae
Martine Maïbèche-Coisne, S. Longhi, E. Jacquin-Joly, Caroline Brunel, M.P. Egloff, L. Gastinel, C. Cambillau, M. Tegoni, Patricia Nagnan-Le Meillour
European Journal of Biochemistry 258:768-774 (1998)
Réseau
National
- Cyrille Mathieu & Denis Gerlier
- CIRI, Lyon
- Pierre-Yves Lozach
- IVPC, Lyon
- Andrey Kajava
- CRBM, Montpellier
- Patrick Fourquet
- CRCM, Marseille
- Joseph Chamieh & Hervé Cottet
- IBMM, Montpellier
- Jean-Guy Berrin
- BBF, Marseille
- Yacine Graba
- IBDM, Marseille
- Pierre-Olivier Vidalain
- CIRI, Lyon
- Vincent Lotteau
- P4 « Jean- Merieux », Lyon
- Mustapha Si-Tahar
- CEPR, Tours
- Samir Messaoudi
- CoSMIT, École Polytechnique, Palaiseau
- Nicolas Tsapis
- Institut Galien, Paris-Saclay
- Tâp Ha-Duong
- Université Paris-Saclay
- Lou Safra
- CEVIPOF, Centre de Recherches Politiques de Sciences Po
- Thierry Desnos
- BIAM, Cadarache
International
- Roberta Pierattelli
- CERM, Florence, IT
- Stefania Brocca & Rita Grandori
- University of Milano-Bicocca, IT
- Vladimir Uversky
- University of South Florida, US
- Samrat Mukhopadyay
- IISER, Mohali, India
- Malte Drescher & Annalisa Pierro
- University of Konstanz, Germany
- Peter Tompa
- VIB, VUB, Belgium
Financement